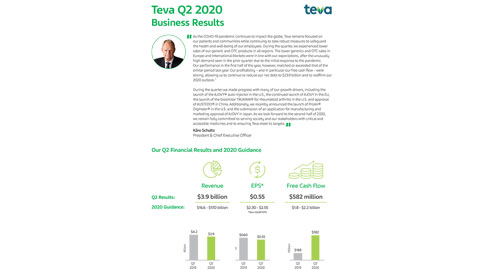
Teva settles with Roche to launch first Rituxan biosimilar in US - BioProcess InternationalBioProcess International

Teva partners with Just Biotherapeutics, ramping up biologics productivity - BioProcess InsiderBioProcess International

Teva two: FDA approves Celltrion-made Herceptin biosimilar - BioProcess InternationalBioProcess International

Sanofi begins work on succession plan; FDA issues warning letters to Lupin's US, India units | Radio Compass Blog

Teva confident in September approval for migraine drug despite latest Celltrion plant citation | FiercePharma