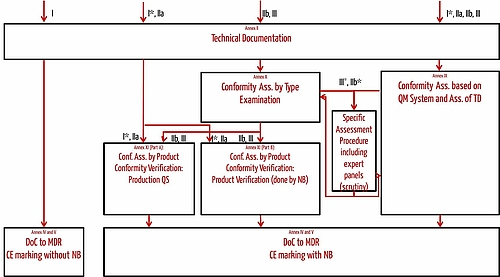
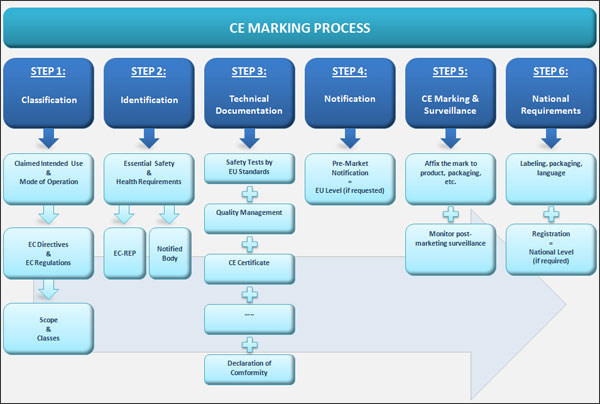
Classification Of Medical Devices And Their Routes To CE Marking – Clever Compliance Support - Compliance system and CE marking information
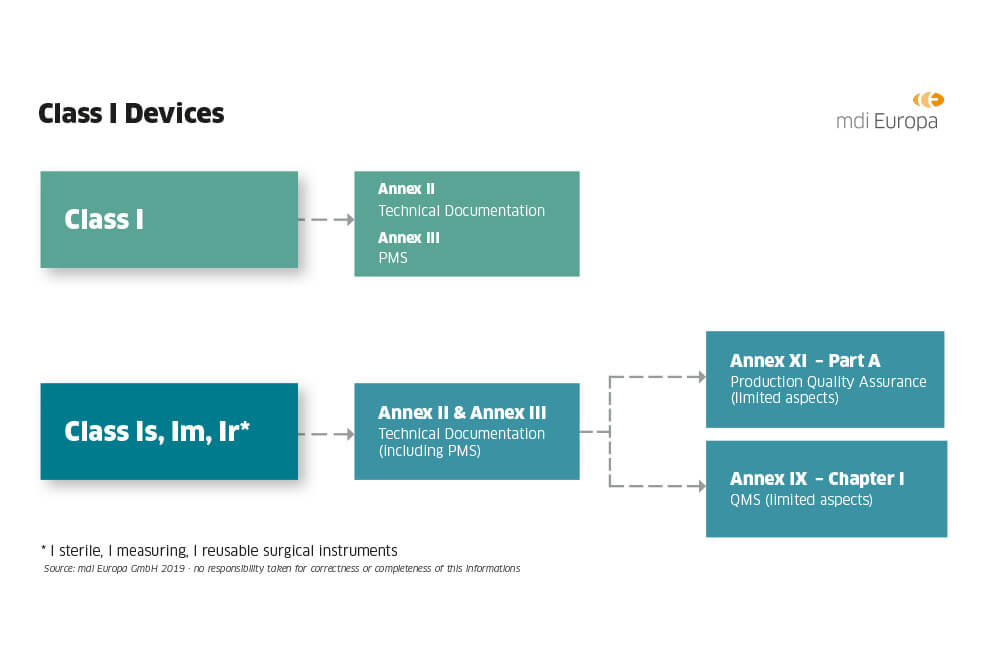
Guide on Class I (Is/Im) MDD- Medical Devices CE marking (mark) & European (EU) Authorized Representative service
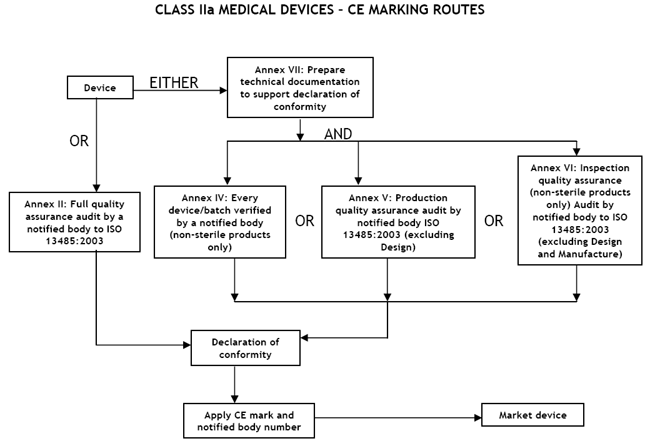
Guide on Class IIa MDD- Medical Devices CE marking (mark) & European (EU) Authorized Representative service
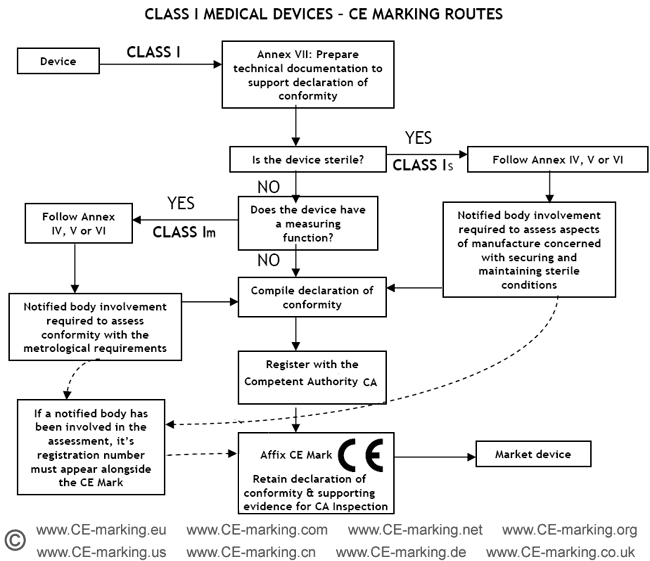
Guide on Class I (Is/Im) MDD- Medical Devices CE marking (mark) & European (EU) Authorized Representative service

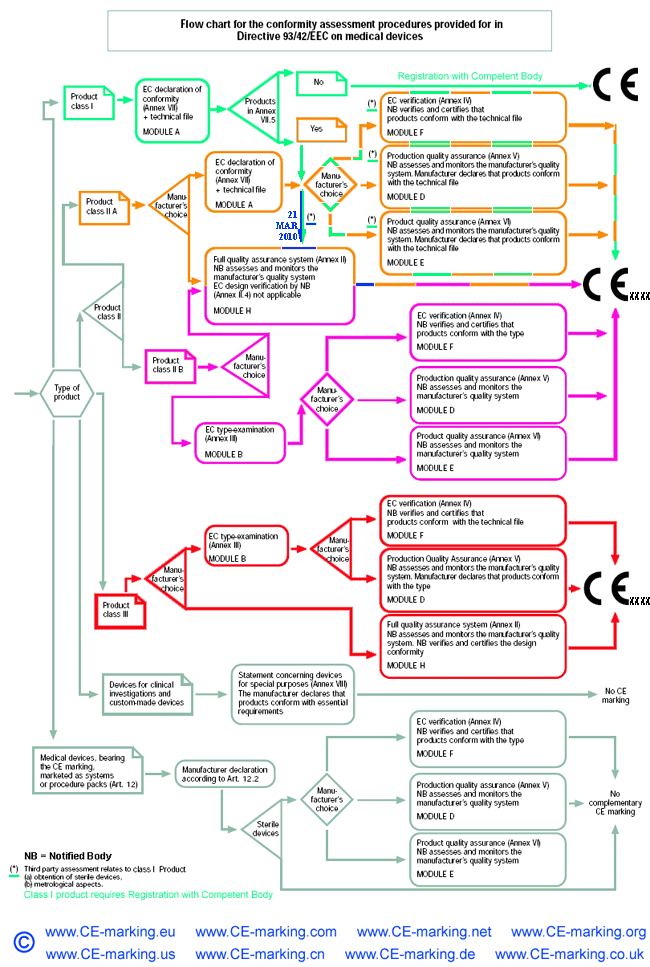
Regulatory bodies and their roles associated with medical devices and wound dressings - ScienceDirect